Aluminum is a light metal with low density (2.79/cm3), good strength and excellent plasticity. As for aluminum alloy, the strength of super-hard aluminum alloy can reach 600Mpa, and the tensile strength of ordinary hard aluminum alloy can reach 200-450Mpa, which is much higher than steel in steel. Therefore, aluminum and aluminum alloy are widely used in machinery manufacturing.
The conductivity of aluminum is second only to silver and copper, so aluminum is used in the manufacture of various conductors. Aluminum also has a good thermal conductivity that can be used as a variety of heat dissipating materials. Besides, aluminum has good corrosion resistance and excellent plasticity, and is suitable for various pressure processing.
Aluminum alloy
Aluminum alloy can be divided into the deformed aluminum alloy and the cast aluminum alloy according to the processing method.
The deformed aluminum alloy can be further divided into a non-heat treatable reinforced aluminum alloy and a heat treatable reinforced aluminum alloy. Non-heat-treated reinforced aluminum alloy cannot improve the mechanical properties by heat treatment, and can only be strengthened by cold working deformation. It mainly includes high-purity aluminum, industrial high-purity aluminum, industrial pure aluminum and rust-proof aluminum. The heat-treatable reinforced aluminum alloy can be improved in mechanical properties by heat treatment such as quenching and aging, and can be classified into hard aluminum, wrought aluminum, super-hard aluminum, and special aluminum alloy. The aluminum alloy can be heat treated to obtain good mechanical properties, physical properties and corrosion resistance.
Cast aluminum alloy can be divided into aluminum-silicon alloy, aluminum-copper alloy, aluminum-magnesium alloy and aluminum-zinc alloy according to chemical composition. Cast aluminum alloy is classified into four types according to the main elements other than aluminum in the composition: silicon, copper, magnesium and zinc.

Pure aluminum products
Pure aluminum products are divided into two categories: smelting and pressure processing. The former is represented by chemical composition Al, and the latter is represented by LG (aluminum, industrial). The aluminum sputtering target is a kind of pure aluminum product.
Pressure processing aluminum alloy
Aluminum alloy pressure processing products are divided into seven categories: rustproof (LF), hard (LY), forged (LD), superhard (LC), coated (LB), special (LT) and brazed (LQ). The state of the commonly used aluminum alloy material is three types of annealing (M igniter), hardening (Y), and hot rolling (R).
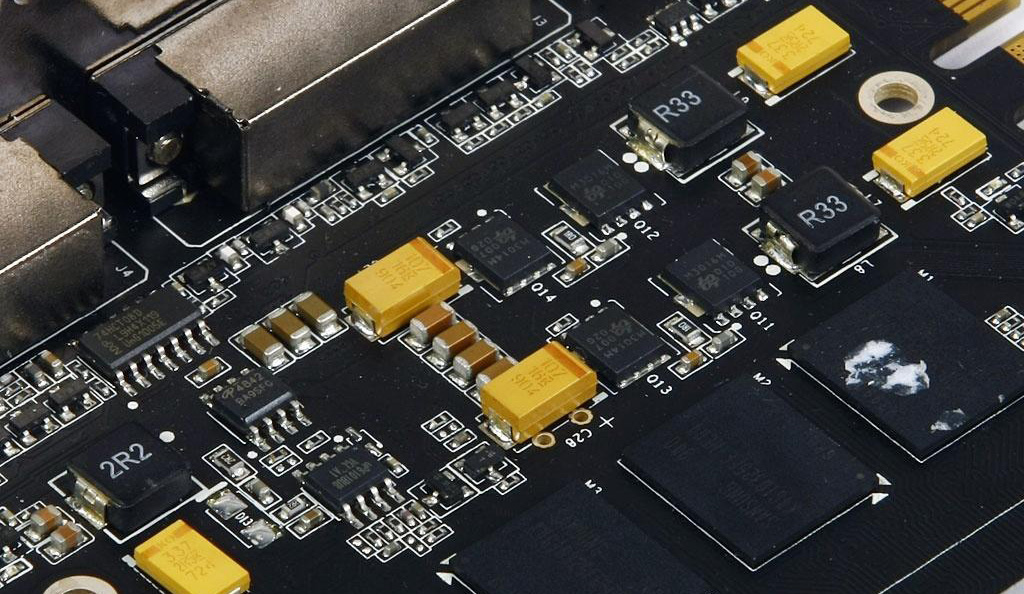
Aluminum sputtering target
The aluminum sputtering target is one of the sputtering targets used in the vacuum coating industry, and is therefore called aluminum sputtering target. The aluminum target is obtained after a series of processing of high-purity aluminum. It is available in a specific size and shape, which is mounted on a vacuum coater to form a film on the surface of the substrate by sputtering.
Please visit https://www.sputtertargets.net/ for more information.